
News
Oct . 02, 2024 13:18 Back to list
CE Certification for a Polymer Derived from Amino Acids and Its Applications
CE Certification for a Polymer of Amino Acids Significance and Applications
In the world of materials science and biotechnology, polymers derived from amino acids have gained considerable attention for their unique properties and vast applications. These polymers hold promise in various fields, such as medicine, pharmaceuticals, and the food industry. However, ensuring their safety and efficacy for consumer use is essential, and this is where CE certification plays a critical role.
Understanding CE Certification
CE certification is a mark that indicates a product's compliance with European health, safety, and environmental protection standards. It is a mandatory requirement for certain products sold within the European Economic Area (EEA). For polymers of amino acids, achieving CE certification involves a thorough evaluation process that assesses the product's safety, quality, and performance. This certification assures consumers and manufacturers that the product meets necessary European regulations and is safe for its intended use.
The Importance of Amino Acid Polymers
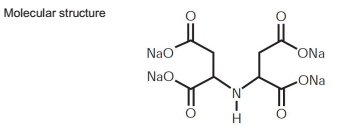
Polymers of amino acids, often referred to as polyamino acids or polypeptides, are versatile materials known for their biocompatibility and biodegradability. These features make them particularly appealing for applications in drug delivery systems, tissue engineering, and biopharmaceuticals. For example, N,N-dimethylaminoethyl methacrylate (DMAEMA) and glycine-based polymers have been researched for their potential in formulating innovative drug delivery systems that can release pharmaceuticals in a controlled manner.
Moreover, due to their natural origins, these polymers can minimize adverse reactions in biological environments, making them suitable for various medical applications, including sutures, implants, and scaffolds for cell growth.
ce certification a polymer of amino acid
Achieving CE Certification for Amino Acid Polymers
Obtaining CE certification involves multiple steps, beginning with compliance with relevant European directives. Depending on the application, these may include the Medical Device Regulation (MDR) or the REACH regulation concerning the Registration, Evaluation, Authorisation, and Restriction of Chemicals.
The process typically includes 1. Risk Assessment Identifying potential hazards associated with the polymer and its applications, ensuring that any risks are controlled. 2. Testing and Evaluation Conducting rigorous testing to evaluate the safety, efficacy, and performance of the polymer in real-world applications. 3. Documentation Compiling a technical file that includes all relevant data, from production processes to testing results, to demonstrate compliance with the requisite standards. 4. Notified Body Involvement Collaborating with a notified body, an organization designated by the national authorities to assess the conformity of certain products before they can be marketed.
Challenges and Future Perspectives
Despite the benefits of CE certification, manufacturers of amino acid polymers face challenges in navigating the complexities of regulation and compliance. The cost and time associated with obtaining CE certification can be significant, particularly for small and medium-sized enterprises.
Nevertheless, the market potential for these polymers is substantial, and their applications in drug delivery, regenerative medicine, and other fields continue to expand. As research advances and innovations emerge, the demand for CE-certified amino acid polymers is likely to grow, promoting safer and more effective products.
In conclusion, CE certification for polymers of amino acids is not just a regulatory hurdle; it is a vital process that ensures safety, efficacy, and quality in the marketplace. As the biopolymer industry evolves, the adherence to these standards will foster trust and promote the development of innovative solutions that can significantly impact the health and wellbeing of society. With continued investment in research and compliance processes, the future looks promising for this exciting field.
-
Polyaspartic Acid Salts in Agricultural Fertilizers: A Sustainable Solution
NewsJul.21,2025
-
OEM Chelating Agent Preservative Supplier & Manufacturer High-Quality Customized Solutions
NewsJul.08,2025
-
OEM Potassium Chelating Agent Manufacturer - Custom Potassium Oxalate & Citrate Solutions
NewsJul.08,2025
-
OEM Pentasodium DTPA Chelating Agent Supplier & Manufacturer High Purity & Cost-Effective Solutions
NewsJul.08,2025
-
High-Efficiency Chelated Trace Elements Fertilizer Bulk Supplier & Manufacturer Quotes
NewsJul.07,2025
-
High Quality K Formation for a Chelating Agent – Reliable Manufacturer & Supplier
NewsJul.07,2025
