
News
Sep . 24, 2024 05:14 Back to list
Understanding the Role and Importance of Chelating Agents in Chemistry
Definition and Importance of Chelating Agents
Chelating agents, also known as ligands, are molecules capable of forming multiple coordination bonds with a single metal ion. This ability to bind metal ions through multiple sites makes chelating agents crucial in various chemical, biological, and environmental processes. The term chelate is derived from the Greek word chēlē, meaning claw, which aptly describes how these agents grasp or encapsulate metal ions, effectively grabbing them.
Definition and Importance of Chelating Agents
In the medical field, chelating agents are often utilized in the treatment of metal poisoning. For instance, agents like dimercaprol and EDTA are used to bind toxic metals such as lead or mercury in the body, facilitating their excretion and minimizing harmful effects. This therapeutic use highlights the importance of chelating agents in detoxification and recovery from heavy metal exposure.
definition of chelating agent
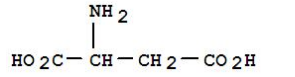
In agriculture, chelating agents enhance the bioavailability of essential nutrients in soil. Many metal ions, such as iron and manganese, can become insoluble and unavailable to plants due to their interaction with other soil components. Chelating agents help maintain these nutrients in a soluble form, allowing for better absorption by plant roots. This application not only improves crop yield but also promotes healthier plant growth.
Moreover, in industrial applications, chelating agents are crucial for processes such as water treatment, metal recovery, and the stabilization of metal-containing products. For instance, in the textile industry, chelating agents are employed to prevent metal ions from interfering with dyeing processes, ensuring even color application and preventing discoloration.
Despite their advantages, the use of chelating agents must be carefully managed, as some synthetic agents can have adverse environmental effects. Research into biodegradable chelators and the development of greener alternatives has become increasingly important.
In conclusion, chelating agents are vital components in various sectors, from healthcare to agriculture and industry. Their ability to bind and transport metal ions effectively makes them indispensable in promoting health, enhancing agricultural productivity, and improving industrial processes. Understanding their function and applications continues to be essential for advancements in science and technology.
-
Polyaspartic Acid Salts in Agricultural Fertilizers: A Sustainable Solution
NewsJul.21,2025
-
OEM Chelating Agent Preservative Supplier & Manufacturer High-Quality Customized Solutions
NewsJul.08,2025
-
OEM Potassium Chelating Agent Manufacturer - Custom Potassium Oxalate & Citrate Solutions
NewsJul.08,2025
-
OEM Pentasodium DTPA Chelating Agent Supplier & Manufacturer High Purity & Cost-Effective Solutions
NewsJul.08,2025
-
High-Efficiency Chelated Trace Elements Fertilizer Bulk Supplier & Manufacturer Quotes
NewsJul.07,2025
-
High Quality K Formation for a Chelating Agent – Reliable Manufacturer & Supplier
NewsJul.07,2025
