
News
Aug . 20, 2024 14:02 Back to list
EDTA Iron Chelation Solutions for Efficient Metal Recovery and Environmental Safety
The Role of EDTA in Iron Chelation Overview of Manufacturing Processes
Iron, despite its essential role in numerous biological functions, can become toxic in excess. This is where chelation therapy comes into play, employing compounds like EDTA (ethylenediaminetetraacetic acid) to bind excess iron in the body and facilitate its excretion. The manufacturing of EDTA and its derivatives suitable for iron chelation is a crucial industry, influencing both healthcare and broader industrial applications.
Understanding EDTA
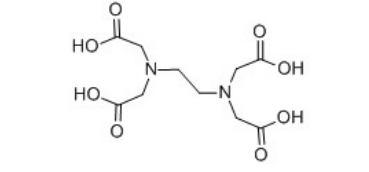
EDTA is a synthetic amino polycarboxylic acid that efficiently binds to metal ions, forming stable, soluble complexes. First synthesized in the 1950s, it quickly found applications in medicine, agriculture, and various industrial sectors. In particular, its ability to chelate iron makes it invaluable for treating conditions like hemochromatosis, where the body accumulates excess iron, potentially leading to organ damage.
The iron chelation process using EDTA occurs through the formation of a stable complex that is then excreted in urine, reducing iron overload in patients
. This process not only alleviates symptoms but also prevents long-term complications associated with iron toxicity.Manufacturing EDTA
The production of EDTA is a coordinated effort that combines chemical engineering with stringent quality control measures. The primary synthesis route involves the reaction of ethylenediamine with chloroacetic acid, followed by a series of hydrolysis steps to yield the final EDTA product. Factories must maintain rigorous operational standards to ensure purity and efficacy, as even small impurities can significantly alter the chelating properties of EDTA.
1. Raw Material Sourcing The manufacturing process begins with sourcing high-purity raw materials. Ethylenediamine and chloroacetic acid must meet strict specifications to ensure that the final EDTA product is safe and effective for therapeutic use.
edta iron chelation factory
2. Reaction Process The core of the manufacturing process involves controlled chemical reactions to produce the EDTA precursor. This stage requires precise temperature and pH control to maximize yield and minimize side reactions. Continuous monitoring and automation technologies are increasingly being integrated to enhance production efficiency.
3. Purification After synthesis, the EDTA must be purified to remove by-products and any unreacted raw materials. Techniques like crystallization, filtration, and chromatography are commonly employed in this stage. Advanced quality control laboratories are essential to test the batch for purity and functionality.
4. Formulation and Packaging Once purified, EDTA is often formulated into various products for specific applications, whether for medicinal use, agricultural enhancement, or industrial processes. The packaging must also align with regulatory requirements to ensure that the product remains stable and secure until it reaches end-users.
Applications Beyond Medicine
While EDTA's role in iron chelation is paramount in medical applications, its utility extends into agriculture as a micronutrient chelator, aiding in the bioavailability of trace minerals to plants. In industrial contexts, EDTA is used in cleaning products, and as a stabilizing agent in cosmetics and food production, showcasing its versatility.
Conclusion
The manufacturing of EDTA is a sophisticated yet essential process that produces a compound critical for iron chelation therapy and various industrial applications. With ongoing research and advancements in chemical engineering, the efficacy and safety of EDTA products will continue to improve, ensuring that they meet the burgeoning demand in both healthcare and industry. As awareness of iron overload and related disorders grows, the role of EDTA as a chelator will remain vital, highlighting the importance of continued investment in manufacturing excellence and innovation.
-
Polyaspartic Acid Salts in Agricultural Fertilizers: A Sustainable Solution
NewsJul.21,2025
-
OEM Chelating Agent Preservative Supplier & Manufacturer High-Quality Customized Solutions
NewsJul.08,2025
-
OEM Potassium Chelating Agent Manufacturer - Custom Potassium Oxalate & Citrate Solutions
NewsJul.08,2025
-
OEM Pentasodium DTPA Chelating Agent Supplier & Manufacturer High Purity & Cost-Effective Solutions
NewsJul.08,2025
-
High-Efficiency Chelated Trace Elements Fertilizer Bulk Supplier & Manufacturer Quotes
NewsJul.07,2025
-
High Quality K Formation for a Chelating Agent – Reliable Manufacturer & Supplier
NewsJul.07,2025
